Updated April 12, 2025
Understanding how to mine FDA databases for insights is a strategic advantage if you’re bringing a medical device to market or managing one post-approval.
These databases aren’t just regulatory archives. They’re treasure troves of competitive intelligence, predicate data, and submission benchmarks that can help you streamline your premarket submission or fortify your cybersecurity documentation.
At Blue Goat Cyber, we help medical device companies do both—faster, smarter, and with less friction.
Here’s how to make the FDA 510(k) and PMA databases work for you.
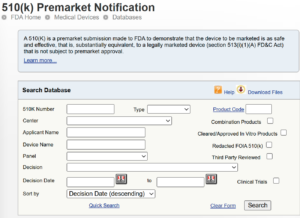
What Is the FDA 510(k) Database, and Why Should You Use It?
Link: FDA 510(k) Database
The 510(k) process allows you to demonstrate that your device is substantially equivalent to a predicate device already on the market.
What You Can Learn:
- Approved predicate devices in your category
- Manufacturer info and submission timelines
- Summaries of substantial equivalence
- Indications for use—great for regulatory positioning
For Cybersecurity Teams:
While 510(k) summaries don’t always detail cybersecurity measures, they do help identify:
- Connected or software-based devices
- Wireless capabilities or cloud integration
- Device classes and product codes associated with higher cybersecurity risk
These are foundational for developing an FDA-ready cybersecurity risk assessment and threat model.
What’s Inside the FDA PMA Database?
Link: FDA PMA Database
Premarket Approval (PMA) is required for Class III devices—those that sustain or support life or pose significant risk.
What You’ll Find:
- Full approval summaries
- Supplements, amendments, and modifications
- FDA review memos and postmarket requirements
Why This Matters:
PMA documentation often includes specific cybersecurity design controls, particularly for:
- Implantable or life-supporting devices
- Networked hospital systems
- Devices using wireless telemetry or remote monitoring
These examples can help you model your own submission and avoid costly delays or RTA (Refuse to Accept) notices.
How to Use These Databases Strategically
Searching these databases shouldn’t be a checkbox activity—it should be part of your regulatory and cybersecurity playbook.
At Blue Goat Cyber, we help clients
- Map cybersecurity strategies based on comparable submissions
- Build SBOMs and secure architectures aligned with known threats
- Prepare FDA documentation that proactively addresses evolving guidance, like the 2023 “Cybersecurity in Medical Devices” update
Pro Tips for Smarter Searches
| Tip | Why It Matters |
| Search by product code | Groups similar devices for competitive insight |
| Use decision date ranges | Filter by recent approvals for the latest regulatory patterns |
| Examine supplemental PMA entries | Track device evolution and postmarket updates |
| Review summary statements | Understand FDA’s focus areas in each submission |
Turn Research into Regulatory Advantage
The FDA’s databases are more than repositories—they’re roadmaps. The challenge is knowing how to interpret them through the lens of cybersecurity, compliance, and market success.
That’s where we come in.
✅ What You Get With Blue Goat Cyber:
- Full-Service premarket submission support
- Cybersecurity documentation aligned to 510(k) or PMA submissions
- Threat modeling and SBOM support tailored to FDA expectations
- Risk management planning for both premarket and postmarket
- Regulatory insights from our experience across dozens of submissions
Schedule a Discovery Session today.


